Spatial Dispersion of Action Potential Duration restitution measured from the surface ECG |
|---|
By A. Mincholé, E. Pueyo and P.Laguna |
Heart rate dependence of action potential duration (APD), also called restitution kinetics, is thought to be critical in activation instability and, therefore, provides relevant information for ventricular arrhythmic risk stratification [1, 2]. The dynamic APD restitution (APDR) curve, measured using the so-called dynamic restitution protocol, quantifies the relationship between the APD and the RR interval (inverse of HR) at steady-state when pacing at different RR values [3, 4]. Individual steeply sloped APDR curves have been reported to play an important role in the development of ventricular arrhythmias. However, heterogeneities in the ventricle lead to non uniform restitution properties, which makes APDR curves present spatial dispersion [5]. Recent studies have suggested that such dispersion in the APDR curves may act as a potent arrhythmogenic substrate [6, 7]. Additionally, increments in that spatial dispersion have been associated with greater propensity to suffer from ventricular tachycardia/fibrillation [8].
The main limitation on the usability of APDR dispersion as a risk index is that its quantification requires invasive procedures. In [9], a complete methodology was proposed to indirectly estimate dispersion of restitution slopes by making only use of the surface electrocardiogram (ECG). We proposed an ECG measure that quantifies dispersion in the dynamic APDR slopes by characterizing the relationship between the distance from T wave peak to T wave end (Tpe) and the RR interval under different stationary conditions. The proposed estimate is ![]() , where
, where ![]() represents the Tpe interval at stationary RR intervals and can be computed as described in [9].
represents the Tpe interval at stationary RR intervals and can be computed as described in [9].
The capability of the proposed estimate to reflect APDR dispersion has been assessed by using a combination of ECG signal processing and computational modeling and simulation. Specifically, ECG recordings of control subjects undergoing a tilt test trial are used to measure that estimate, while its potentiality to provide a quantification of APDR dispersion at tissue level is assessed by using a 2D ventricular tissue simulation.
From this 2D simulation, APDR dispersion, denoted as ![]() , is calculated, and pseudo-ECGs are derived. Estimates of APDR dispersion measured from the pseudo-ECGs (
, is calculated, and pseudo-ECGs are derived. Estimates of APDR dispersion measured from the pseudo-ECGs (![]() ) show to correlate with
) show to correlate with
![]() , being the mean relative error below 5% (see Fig. 1) [9,10].
, being the mean relative error below 5% (see Fig. 1) [9,10].
A comparison of the ECG estimates (![]() ) obtained from tilt test recordings and the
) obtained from tilt test recordings and the
![]() values measured in silico simulations at tissue level show that differences between them are below 20%, which is within physiological variability limits (see Fig. 1) [9]. These results provide evidence that the proposed estimate is a non invasive measurement of APDR dispersion in ventricle.
values measured in silico simulations at tissue level show that differences between them are below 20%, which is within physiological variability limits (see Fig. 1) [9]. These results provide evidence that the proposed estimate is a non invasive measurement of APDR dispersion in ventricle.
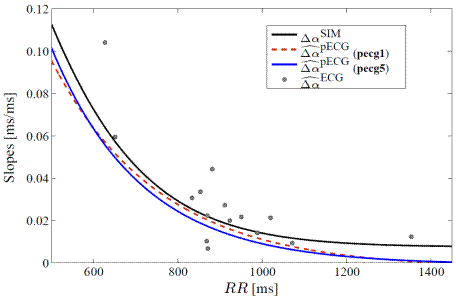
FIGURE 1:
Figure1.- APDR slope dispersion at tissue level ![]() , the proposed estimate measured from the pseudo-ECG in two different sensor positions, pecg1 and pecg5, described in [9, 10]), and the estimates measured from the clinical tilt test ECG recordings.
, the proposed estimate measured from the pseudo-ECG in two different sensor positions, pecg1 and pecg5, described in [9, 10]), and the estimates measured from the clinical tilt test ECG recordings.
A future extension of this work is to test the proposed estimate under pathological conditions. We aim at confirming experimental observations relating enhanced APDR dispersion and arrhythmic risk. By quantifying our proposed ECG index, we seek to assess, on the one hand, whether patients that have suffered arrhythmic events present elevated APDR dispersion and, on the other hand, whether our proposed index is able to predict the onset of arrhythmic episodes.
References
- D. S. Rosenbaum, L. E. Jackson, J. M. Smith, H. Garan, J. N. Ruskin, and C. R. J., “Electrical alternans and vulnerability to ventricular arrhythmias,” N Engl J Med., vol. 330, no. 4, pp. 235–241, 1994.
- M. L. Koller, M. L. Riccio, and R. F. Gilmour Jr, “Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation,” Am J Physiol Heart Circ Physiol, vol. 275, pp. 1635–1642, 1998.
- M. R. Franz, C. D. Swerdlow, L. B. Liem, and J. Schaefer, “Cycle Length Dependence of Human Action Potential Duration In Vivo,” J. Clin. Invest., vol. 82, pp. 972–979, 1988.
- M. L. Riccio, M. L. Koller, and R. F. Gilmour Jr, “Electrical Restitution and Spatiotemporal Organization During Ventricular Fibrillation,” Circ Res., vol. 84, pp. 955–963, 1999.
- K. R. Laurita, S. D. Girouard, and D. S. Rosenbaum, “Modulation of ventricular repolarization by a premature stimulus: Role of epicardial dispersion of repolarization kinetics demonstrated by optical mapping of the intact guinea pig heart,” Circ Res, vol. 79, pp. 493–503, 1996.
- M. P. Nash, C. P. Bradley, P. M. Sutton, R. H. Clayton, P. Kallis, M. P. Hayward, D. J. Paterson, and P. Taggart, “Whole heart action potential duration restitution properties in cardiac patients: a combined clinical and modelling study,” Experimental physiology, vol. 91, no. 2, pp. 339–54, 2006.
- R. Coronel, F. J. G. Wilms-Schopman, T. Opthof, and M. J. Janse, “Dispersion of repolarization and arrhythmogenesis,” Heart Rhythm, vol. 6, no. 4, pp. 537–543, 2009.
- [141] H. Pak, S. Hong, G. Hwang, H. Lee, S. Park, J. Ahn, Y. Moo Ro, , and Y. Kim, “Spatial dispersion of action potential duration restitution kinetics is associated with induction of ventricular tachycardia/fibrillation in humans,” Journal of cardiovascular electrophysiology, vol. 15, no. 12, pp. 1357–63, 2004.
- A. Mincholé, E. Pueyo, J.F. Rodríguez, E. Zacur, M. Doblaré, P. Laguna. “Quantification of restitution dispersion from the dynamic changes of the T wave peak to end, measured at the surface ECG”. IEEE Trans Biomed Eng, In press, 2010 doi:10.1109/TBME.2010.2097597.
- A. Mincholé, E. Pueyo, J.F. Rodríguez, E. Zacur, M. Doblaré, P. Laguna, “Evaluation of a method for quantification of restitution dispersion from the surface ECG”, in Proc. Computers in Cardiology, vol. 36, IEEE Computer Society Press, Belfast (UK), 2010.