High-Resolution 12-lead 48-hours continuous ECG from end-stage renal disease Patients: during and after hemodialysis session. |
|---|
IDENTIFICATION : E-HOL-12-0051-016 |
Study Design:
The goal of study from which these ECGs were recorded was to test the hypothesis that measurement of cardiac repolarization heterogeneity in response to dialysis, can be used to stratify End Stage Renal Disease (ESRD)patients in terms of risk for sudden arrhythmic death and thereby determine which patients may benefit most from ICD placement.
Fifty-one ESRD patients with significant risk for sudden arrhythmic death were enrolled in one University of Rochester affiliated out-patient dialysis centers. Financial incentive was offered to patients for their time and effort. Information about patient cardiac history and current drug therapies was recorded under HIPPA regulation. After the consent forms was communicated and signed, the patients entered the protocol during their next dialysis session. The study design includes 3 periods: A, baseline; B, HD period; C: post-HD. Clinical information such as age, gender, BMI and number of months on dialysis are recorded as well. .
The figure below provides information related to the study characteristics: the upper panel includes the list of clinical parameters measured during the study and availble in the THEW in addtion to age, gender and list of medication taken by the patient.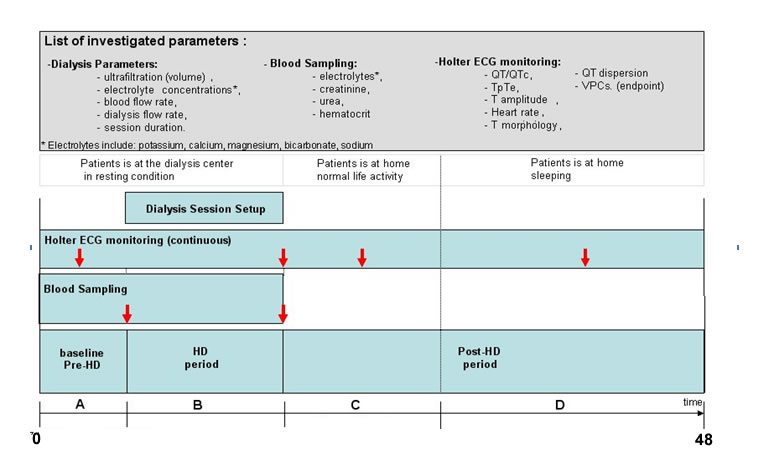
Enrollment criteria
End stage renal disease (ESRD) patients with high risk for cardiac arrhythmias and sudden cardiac death.
Study Population:
ECG Number of Leads: 12 lead standard configuration
ECG Sampling Frequency : 1000Hz
ECG Amplitude Resolution: 0.5 uV
File Naming Convention: Each filename is composed by a unique "ID".
Clinical Information: This database includes hundreds of clinical factors related to the hemodialysis setup, Information about patients blood pressure, medication, electrolytes and many more are provided in this database.