Newsletter Issue 12, July 2011 |
|---|
Risk Stratification of End Stage Renal Disease patients: continuous 48-hour monitoring |
- Editorial: risk stratification of ESRD patients
- FDA/THEW New Private-Public-Partnership Agreement
- New Database: E-HOL-12-0051-016.
- Spatial Dispersion of Action Potential Duration restitution measured from the surface ECG.
- THEW Team: Michelle Richardson
 Editorial: Risk stratification of End Stage Renal Disease Patients
Editorial: Risk stratification of End Stage Renal Disease Patients
by JP. Couderc, PhD, MBA
End Stage Renal Disease (ESRD) is a costly and disabling condition that disproportionately affects racial/ethnic minority populations and is associated with a high mortality rate (230 per 1000 patients/year). The number of ESRD patients has increased at an average yearly rate of 3-4% between 1996 and 2005 in the United States. In 2000, there were ~281,000 US dialysis patients, with a projection of ~520,000 dialysis patients by 2010. Today, there are approximately half a million people in the US with ESRD on hemodialysis and the annual mortality rate among these patients is close to 25%. Cardiac disease is implicated in as many as 44% of these deaths. Amongst those, cardiac arrhythmias represent 61% of all cardiac deaths.
Several prospective multicenter trials (MADIT I/II, AVID, CASH and CIDS) have demonstrated the benefit of implantable cardiodefibrillator (ICD) therapy for the reduction of sudden cardiac death (SCD) in high risk patients. A meta-analysis of three trials suggested better survival with ICDs with a 28% reduction of risk (p<0.0006). Unfortunately, dialysis patients were, in general, excluded from the large randomized trials. Retrospective studies suggested important benefits of ICDs in dialysis patients. Based on a group of 5,582 patients with acute myocardial infarction in which 460 dialysis patients (~8%) were implanted with ICDs, Herzog et al. reported a 42% reduction in cardiac risk. The study also emphasized the underutilization of ICDs in this population. This observation was consistent with the results from Voigt et al. in 2004 describing ICD implantation in a group of survivors of cardiac arrest between 1996 and 2001: 31% were implanted whereas only 18% of dialysis patients surviving cardiac arrest received an ICD. Because the dialysis patients are at high risk for SCD, it is not surprising that most studies emphasize that these patients have the most to gain from receiving life-saving ICD therapies. At present, there are no specific clinical guidelines for the prescription of ICDs in ESRD patients and clinicians need risk-stratification methods to alter the abysmal outcome these patients are facing.
Thus, the Telemetric and Holter ECG Warehouse proposes a new set of continuous 48-hour high-definition (1000Hz) recordings acquired in ESRD patients in order to provide the opportunity to scientists to investigate whether there is information in the surface ECGs that could help better identify the high-risk patients with ESRD using their surface electrocardiograms. Thirteen months follow up on a set of 50 patients recorded during hemodialysis and for a period of two days are available. Eleven patients died during the study follow up (4 had cardiac deaths, while the 8 remaining deaths were not fully documented). See our description of this new database
here
.
In addition this quarter, we are glad to welcome numerous new organizations to our initiave. Two companies: Medial Research Inc., and Philips Health Care and five not-for-profit organizations have joined our initiative this past two months. We hope you will enjoy this Newsletter and our section related to new technologies that was provided by Dr. Mincholeand collaborators from University of Zaragoza (Spain). Anna describes an interesting method to measure spatial dispersion of APD restitution from surface ECGs.
FDA/THEW Private-Public Partnership

On June 23rd, 2011, the partnership between the FDA and the THEW has been renewed after a successful first three years of collaboration. This second agreement has been signed by Dr. Janet Woodcock (Director of the Center for Drug Evaluation and Research, FDA). A copy of this agreement is available on the THEW webite. In addition to the initial claim of the former agreement, this new version has been updated to include various achievements of the past three years and the expected goals for the upcoming years. A description of the partnership is available on the FDA website at the FDA website for Public Private Partnerships (PPP). click here to access this website.
The goal of the study from which this new set of Holter ECG signals were recorded was to test the hypothesis that measurement of cardiac repolarization heterogeneity in response to dialysis, can be used to stratify End Stage Renal Disease (ESRD) patients in terms of risk for sudden arrhythmic death and thereby determine which patients may benefit most from ICD placement.
Fifty-one ESRD patients with significant risk for sudden arrhythmic death were enrolled in one University of Rochester affiliated out-patient dialysis centers. Information about patient cardiac history and current drug therapies were recorded following HIPAA regulation. After the consent forms were communicated and signed, the patients entered the protocol during their next dialysis session. The study design includes 3 periods: A, baseline; B, HD period; C: post-HD. Clinical information such as age, gender, BMI and number of months on dialysis were recorded.
The figure below provides information related to the study characteristics: the upper panel includes the list of clinical parameters measured during the study and available in the THEW in addition to more than 100 clinical factors related to the hemodialysis setup, patients electrolytes, fluid remove, etc. Full description for the database here .
The inclusion/exclusion of patients were as follows:
- Enrollment criteria•End stage renal disease (ESRD) patients with high risk for cardiac arrhythmias and sudden cardiac death.
- Age greater than 40 years AND
- Confirmed history of hypertension requiring treatment OR
- Confirmed history of diabetes requiring treatment
- Exclusion criteria
- Subject with class I antiarrhythmic, according to cardiologist evaluation
- Subject with pacemaker
- Subject with ICD device
- Subject with cardiac resynchronization therapy (CRT) device
- Subject has a history of chronic atrial fibrillation
- Female subject of childbearing potential not using medically prescribed contraceptive measures.
- Subject participating in other clinical trials
- Subject unable to cooperate with the protocol due to dementia, psychological, or other related reason.
- Subject who refuses to sign the consent for participation.
- Number of patients enrolled : N=51
- Enrollment: 2/13/2009 to 06/18/2010
- Duration of the study: 6 months
- Patient follow-up: 13 months after completion of enrollment
- Number of deaths: 11 patients (8 patients completed the study, 4 had cardiac-related death)
Study Population:
ECG Characteristics: Number of Leads: 12 lead standard configuration; ECG Sampling Frequency : 1000Hz; ECG Amplitude Resolution: 0.5 uV
TECHNICAL CORNER: Spatial Dispersion of Action Potential Duration restitution measured from the surface ECG
By A. Mincholé, E. Pueyo and P.Laguna
Heart rate dependence of action potential duration (APD), also called restitution kinetics, is thought to be critical in activation instability and, therefore, provides relevant information for ventricular arrhythmic risk stratification [1, 2]. The dynamic APD restitution (APDR) curve, measured using the so-called dynamic restitution protocol, quantifies the relationship between the APD and the RR interval (inverse of HR) at steady-state when pacing at different RR values [3, 4]. Individual steeply sloped APDR curves have been reported to play an important role in the development of ventricular arrhythmias. However, heterogeneities in the ventricle lead to non uniform restitution properties, which makes APDR curves present spatial dispersion [5]. Recent studies have suggested that such dispersion in the APDR curves may act as a potent arrhythmogenic substrate [6, 7]. Additionally, increments in that spatial dispersion have been associated with greater propensity to suffer from ventricular tachycardia/fibrillation [8].
The main limitation on the usability of APDR dispersion as a risk index is that its quantification requires invasive procedures. In [9], a complete methodology was proposed to indirectly estimate dispersion of restitution slopes by making only use of the surface electrocardiogram (ECG). We proposed an ECG measure that quantifies dispersion in the dynamic APDR slopes by characterizing the relationship between the distance from T wave peak to T wave end (Tpe) and the RR interval under different stationary conditions. The proposed estimate is ![]() , where
, where ![]() represents the Tpe interval at stationary RR intervals and can be computed as described in [9].
represents the Tpe interval at stationary RR intervals and can be computed as described in [9].
The capability of the proposed estimate to reflect APDR dispersion has been assessed by using a combination of ECG signal processing and computational modeling and simulation. Specifically, ECG recordings of control subjects undergoing a tilt test trial are used to measure that estimate, while its potentiality to provide a quantification of APDR dispersion at tissue level is assessed by using a 2D ventricular tissue simulation.
From this 2D simulation, APDR dispersion, denoted as ![]() , is calculated, and pseudo-ECGs are derived. Estimates of APDR dispersion measured from the pseudo-ECGs (
, is calculated, and pseudo-ECGs are derived. Estimates of APDR dispersion measured from the pseudo-ECGs (![]() ) show to correlate with
) show to correlate with ![]() , being the mean relative error below 5% (see Fig. 1) [9,10].
, being the mean relative error below 5% (see Fig. 1) [9,10].
A comparison of the ECG estimates (![]() ) obtained from tilt test recordings and the
) obtained from tilt test recordings and the ![]() values measured in silico simulations at tissue level show that differences between them are below 20%, which is within physiological variability limits (see Fig. 1) [9]. These results provide evidence that the proposed estimate is a non invasive measurement of APDR dispersion in ventricle.
values measured in silico simulations at tissue level show that differences between them are below 20%, which is within physiological variability limits (see Fig. 1) [9]. These results provide evidence that the proposed estimate is a non invasive measurement of APDR dispersion in ventricle.
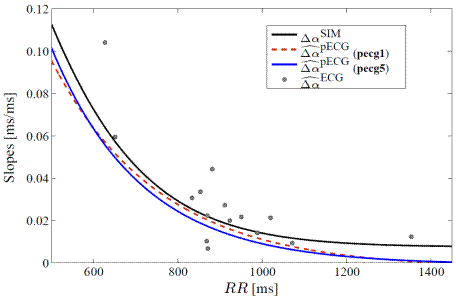
FIGURE 1:
Figure1.- APDR slope dispersion at tissue level ![]() , the proposed estimate measured from the pseudo-ECG in two different sensor positions, pecg1 and pecg5, described in [9, 10]), and the estimates measured from the clinical tilt test ECG recordings.
, the proposed estimate measured from the pseudo-ECG in two different sensor positions, pecg1 and pecg5, described in [9, 10]), and the estimates measured from the clinical tilt test ECG recordings.
A future extension of this work is to test the proposed estimate under pathological
conditions. We aim at confirming experimental observations relating enhanced APDR
dispersion and arrhythmic risk. By quantifying our proposed ECG index, we seek to assess,
on the one hand, whether patients that have suffered arrhythmic events present elevated
APDR dispersion and, on the other hand, whether our proposed index is able to predict the
onset of arrhythmic episodes.
References
- D. S. Rosenbaum, L. E. Jackson, J. M. Smith, H. Garan, J. N. Ruskin, and C. R. J., “Electrical alternans and vulnerability to ventricular arrhythmias,” N Engl J Med., vol. 330, no. 4, pp. 235–241, 1994.
- M. L. Koller, M. L. Riccio, and R. F. Gilmour Jr, “Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation,” Am J Physiol Heart Circ Physiol, vol. 275, pp. 1635–1642, 1998.
- M. R. Franz, C. D. Swerdlow, L. B. Liem, and J. Schaefer, “Cycle Length Dependence of Human Action Potential Duration In Vivo,” J. Clin. Invest., vol. 82, pp. 972–979, 1988.
- M. L. Riccio, M. L. Koller, and R. F. Gilmour Jr, “Electrical Restitution and Spatiotemporal Organization During Ventricular Fibrillation,” Circ Res., vol. 84, pp. 955–963, 1999.
- K. R. Laurita, S. D. Girouard, and D. S. Rosenbaum, “Modulation of ventricular repolarization by a premature stimulus: Role of epicardial dispersion of repolarization kinetics demonstrated by optical mapping of the intact guinea pig heart,” Circ Res, vol. 79, pp. 493–503, 1996.
- M. P. Nash, C. P. Bradley, P. M. Sutton, R. H. Clayton, P. Kallis, M. P. Hayward, D. J. Paterson, and P. Taggart, “Whole heart action potential duration restitution properties in cardiac patients: a combined clinical and modelling study,” Experimental physiology, vol. 91, no. 2, pp. 339–54, 2006.
- R. Coronel, F. J. G. Wilms-Schopman, T. Opthof, and M. J. Janse, “Dispersion of repolarization and arrhythmogenesis,” Heart Rhythm, vol. 6, no. 4, pp. 537–543, 2009.
- [141] H. Pak, S. Hong, G. Hwang, H. Lee, S. Park, J. Ahn, Y. Moo Ro, , and Y. Kim, “Spatial dispersion of action potential duration restitution kinetics is associated with induction of ventricular tachycardia/fibrillation in humans,” Journal of cardiovascular electrophysiology, vol. 15, no. 12, pp. 1357–63, 2004.
- A. Mincholé, E. Pueyo, J.F. Rodríguez, E. Zacur, M. Doblaré, P. Laguna. “Quantification of restitution dispersion from the dynamic changes of the T wave peak to end, measured at the surface ECG”. IEEE Trans Biomed Eng, In press, 2010 doi:10.1109/TBME.2010.2097597.
- A. Mincholé, E. Pueyo, J.F. Rodríguez, E. Zacur, M. Doblaré, P. Laguna, “Evaluation of a method for quantification of restitution dispersion from the surface ECG”, in Proc. Computers in Cardiology, vol. 36, IEEE Computer Society Press, Belfast (UK), 2010.
