Newsletter Issue 8, May 2010 |
|---|
Thorough Holter QT studies, LQTS database and NHLBI Cooperation |
- Editorial
- NIH/NHLBI joins the THEW Steering Committee
- THEW new database: 24-hour Holter recordings from genotyped LQTS patients
- FDA and thorough Holter QT studies: development
- THEW Scientific Session in Belfast
- Profiling the THEW Team: Jean Xia, software engineer and PhD student
Editorial by JP Couderc, PhD
This past month we have started a cooperative agreement with the National Heart Lung and Blood Institute (NHLBI). This agreement is a mechanism for our initiative to involve substantial Federal scientific and programmatic support from the NHLBI. The first step in this support is the joining of two scientific staffs within our steering committee to help us guide and coordinate our project activities (see article below). Interestingly, ECG-related research has been strong in the history of the NIH. A personal review of current NIH-funded activities related to ECG has revealed that this reasearch arena is very active. Indeed, the average number of NIH awards related to the development of ECG technologies or relying on ECG information awarded each year between 2000 and 2009 was 153 (based on the Research Portfolio Online Reporting tool (RePORt) from the NIH). More interestingly, there is a trend toward an increasing number of this type of award (see figure below).
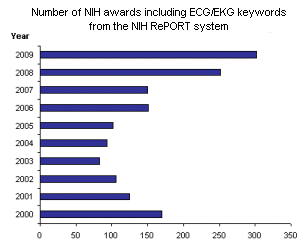
Left figure: number of NIH awards supported each year and related to development of ECG technologies. Note: this does not include the NIH awards that did not specifically focus on ECGs but could have included basic ECG metrics.
One hundred seventy NIH proposals were awarded in the year 2000. In 2003, fewer than 100 awards were granted representing the smallest number of NIH-funded research projects for this current decade.
The largest number of awards during these past 9 years was released last year (2009) and this number may be primiraly driven by the The American Recovery and Reinvestment Act passed on February 17, 2009. Yet, the graph reveals a clear continuous growth in numbers of awards between 2003 and 2008, with a three fold increase from 83 to 252 projects granted by the NIH in 2008. These numbers confirm the very active research currently implemented either for developing ECG-related technologies or relying on ECG information. The rational for the growing interest in ECG technologies is driven by many factors and amongst the reviewed awards one can note research related to cost-effective cardiac markers, the interest for concepts related to personal cardiac safety and monitoring technologies (ambulatory, home-device, etc.).
Related to the activity of our Center during these past couple of months, we received another set of very interesting Holter recordings from patients with the congenital long QT syndrome. Dr. Fabrice Extramania et al. describes below this population and their effort to gather Holter ECG from genotyped LQTS patients during the past 25 years. This data has been received by our groupand it is available in the warehouse for download.
Publications based on THEW data will soon be available. Numerous abstracts based on the data from the THEW were submitted to the Computing in Cardiology meeting held in Belfast this September. The organizers agreed on planning a THEW session during which the most interesting work done with the THEW data will be presented.
From the regulatory side, continuous QT measured from Holter recordings are going to be reviewed at the FDA in the context of Thorough QT studies involving compounds with pronounced effects on the autonomic nervous system. The CDER released information about a CPI (Critical Path Initiative) fellowship award for a two-year project for the development of review processes of three method : beat-to-beat QT, individual heart-rate correction for QTc and RR bin method. The THEW provides the offcial release of this fellowship.
To conclude, growth is a clear trend for this first half of the year 2010 with more data coming in, additional comittment from federal Agency, release of publications from the THEW scientific network and exciting developments at the FDA related to Holter technologies.
Dr. David Lathrop and Dr. Robin Boineau have officially joined the Steering Committee of the Telemetric and Holter ECG Warehouse (THEW). Dr. Lathrop is Deputy Chief of the Heart Failure & Arrhythmia Branch of the Division of Cardiovascular Sciences at the National Heart, Lung, and Blood Institute and Dr. Robin Boineau is Medical Officer at the Cardiology department at the NHLBI.
Dr. Boineau commented on this new cooperation: "David Lathrop and I look forward to joining the THEW as it evolves into the Center for Quantitative Electrocardiography and Cardiac Safety (CES). We look forward to working with Jean-Philippe Couderc and his colleagues in Rochester, our FDA colleagues, and others as the CES becomes a resource for investigators designing and validating analytic methods in quatitative electrocardiography and cardiac safety."
The THEW team welcomes Drs. Boibeau and Lathrop to the THEW Initiative; we are looking forward to their input into the strategic developments of the project in collaboration with other members from the FDA and from the University of Rochester.
THEW new database: 24-hour Holter recordings from genotyped patients with the congential long QT syndrome by Fabrice Extramiana, MD, PhD et al.
Torsades de pointes have been initially described in our institution in the sixties and ever since the interest for QT prolongation-related arrhythmias has been very important in our department.
In the mid eighties, Philippe Coumel initiated the systematic recording of Holter ECGs in patients with a prolonged ventricular repolarization.
This Holter database has been nurtured by Isabelle Denjoy, and after discovery of the underlying genetic basis of the syndrome phenotypic ECG data accumulated over 25 years have been linked with the results of genetic data obtained in LQTS patients (N=480).
This primary care-oriented database contains clinical data (demographics, symptomatic status, and beta-blocker treatment at the time of recording) of genotyped LQTS patients who have had one or repeated Holter ECG recording(s).
Over the last 2 decades, recordings have been obtained with different technologies but mainly with the different versions of the ELA Medical software. Data were initially analog recordings that have been subsequently digitized, and more recently recordings are digital but with different formats.
Thanks to the THEW initiative, all recordings have been reformatted into the ISHNE format. We are proud to share our unique Holter database with the scientific community via the THEW initiative. We are confident that allowing other research groups to use our data will speed up the quest for a better understanding of LQTS and hopefully shorten the time to improved risk stratification and treatment strategies.
Fabrice Extramiana, MD, PhD
Pierre Maison-Blanche, MD
Isabelle Denjoy, MD
Antoine Leenhardt, MD
Lariboisière Hospital arrhythmia unit, Paris 7 University, France
Delays in cardiac repolarization (QT/QTc prolongation) are associated with lethal arrhythmias. Evaluation of the QT/QTc prolonging effects of new compounds has been challenging for drugs with pronounced effects on the autonomic nervous system, which can have secondary effects on cardiac repolarization. To identify drug-induced QT/QTc prolongation while adjusting for drug-induced changes in autonomic tone, scientists and experts have proposed analytic strategies relying on continuous Holter monitor recordings. These methods include: 1) the evaluation of individual QT/RR coupling (QTci); 2) the definition of a normal 24-hour range of QT/RR couplets (QT beat-to-beat), and 3) the design of rate-related referential QT benchmark (RR bin method). These three approaches are considered to be improved analytic strategies to evaluate the effects of a new compound on the QT/QTc interval in situations where the drug also changes the autonomic regulation of the heart. More importantly, these methods can be applied to data recorded in thorough QT studies (TQT studies, adhering to ICH E14 recommendations) if they were designed to use data from continuous 24-hour ECG recordings.
These methods require the analysis of long-term multi-lead continuous recordings, each including on the order of 100,000 cardiac beats on which these measurements are made. The current capacity of the FDA to receive, process, and evaluate this information is insufficient, and the primary objective of the proposed work is to develop these capacities inside the Center for Drug Evaluation and Research (CDER).
The project proposes a two-year research fellowship position at the FDA Campus in Silver Spring, Maryland, for the development of internal quality evaluation, analysis, and collation tools for Holter data submitted to the FDA by pharmaceutical companies. The proposed project would benefit from an individual already familiar with the ECG methods described above, as well as expertise in quantitative electrocardiography. Candidates are invited to participate in the THEW training class (RES950) at University of Rochester Medical Center prior to joining the CDER/FDA.
Official FDA announcement available here in pdf format
We are glad to announce that several databases from the THEW have been used for research activities, and the preliminary publications will be soon released during the next Computing in Cardiology (CinC) meeting. CinC (
www.cinc.org
) is an International Scientific Conference that has been held annually since 1974, it provides a forum for scientists and professionals from the fields of medicine, physics, engineering and computer science to discuss their current research in topics pertaining to computing in clinical cardiology and cardiovascular physiology. Eight abstracts were submitted to the Annual Computing in Cardiology (formerly Computers in Cardiology) meeting from various academic and private partners of the THEW. The CinC organizers agree on including a THEW session in this year program during which the most interesting works base on the THEW database will be presented.
This year the meeting is held in Belfast (UK), it is hosted by the University of Ulster. You can find more information about the meeting at:
http://www.cinc2010.org
.

THEW Team: Jean Xia, Software Engineer
Jean Xia was born in China, and came to the United States to continue her education in 1993. She received her masters degree in Biophysics and Computer Science from the University of Rochester and Rochester Institute Technology in 1996 and 2001, respectively. In 2001, she joined the Clinical Cardiovascular Research Center as a software engineer. During the past nine years, she developed research software and several programs for supporting various researches in Dr. Couderc's lab. Now she is a part-time PhD student in the Department of Electrical and Computer Engineering at University of Rochester. Her responsibilities in the THEW organization are to develop software to support client application, convert ECG files into THEW repository compliant format and ensure technical support to THEW users.