Issue 7
Feb 15,2010
|
Cardiac safety and "Torsades de pointes"
- Editorial
- ICH E14 update by Borje Darpo, PhD, MD
- THEW NEWS
- Hospital providers should be aware of TdPs by B. Drew, RN, PhD, FAHA
- Profiling the THEW Team: Betty Mykins
|
| |
Editorial by JP. Couderc, PhD,MBA
"Torsade de pointes” (TdP), literally translated from French as “a twisting of the points”, describes a ventricular tachycardia with the electrical axis rotating, changing from one direction to another and back again. It is an uncommon polymorphic ventricular tachycardia, which by definition is associated with baseline QT prolongation. First reported in 1966 by Francois Dessertenne in a 80-yr old woman, the specific morphology of the malignant arrhythmias was suspected to be generated by a double competing foci configuration. This hypothesis was confirmed 15 years later in a porcine model where multiple torsades de pointes (TdPs) could be recorded by pacing the heart from both the left and right ventricular sites at a similar but periodically changing rate.[2]
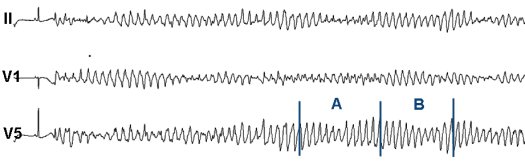
Sotalol-induced TdPs available in the THEW database (click to enlarge). More information about the THEW TdP database can be found here.
Interestingly, the French term "torsade de pointes" has been adopted by the medical community following Dessertenne publication. The correct spelling of "torsade" (with or without an "s") has been the topic of published discussions [1]. The French dictionnary defines a torsade as "a cordon, tie or rope twisted following the shape of an helix or a spiral" without specification about its length or the number of twists required to define one torsade. Desertenne used the same definition of torsade(s) as one can find today in the Robert French dictionary [3]. Based on this definition, one would confidently accept that a "torsade" may be associated with a full arrhythmia tracing regardless of its duration, and "torsades" to multiple separated arrhyhtmias or when there is more than one axis rotating [2]. Interestingly, there is another definition of torsade that can be found in the field of mathematics. The definition of this torsade function differs when it relates to a second or a third dimension. Translating the mathematical definition of a 2D torsade in layman's terms could be as follows: if a ribbon has one side contained within a given plane, a torsade depends on the position of the ribbon borders in reference to this plan. The torsade function must include at least two crossings ensuring that the borders come back to their intitial state. Therefore, the ECG tracing should go through a pattern including both the section A and B of the above figure to fully define one single torsade. In three dimensions, the 2D torsade function is combined with a rotation and the function is linked to the White theorem. This theorem has important implications for molecular biology of DNA where the two borders correspond to the edges of the DNA helix. Now that TdPs have been associated with QTc prolongation, inherited disease and genetic profiling, Dr. Dessertenne would most likely be surprised how appropriate the name was that he selected for this arrhythmia, with or without an "s".
In this issue, we have two contributions related to TdPs and cardiac safety from Dr. Barbara Drew and Dr. Borje Darpo. The article from University of California San Francisco (Dr. Drew) relates to a recent article recently published in Circulation and describing new recommedations about the monitoring of drug-induced torsades de pointes in hospital settings. Dr. Barbara Drew provides an overview of these recommendations highlighting the need for the monitoring of electrocardiographic precursor signs of TdPs. The second article is from Dr. Borje Darpo. Dr. Darpo is Associate Professor of Cardiology at the Karolinska Institute, South Hospital, Stockholm (Sweden), and he has been involved in the writting and release of the ICH E14 documents describing the recommendations for the implementation of thorought QT studies (TQT). These safety studies are implemented to assess whether new drugs carry the potential for prolonging the QTc interval and thus be associated with a risk for torsades de pointes. In this article you will learn about the ICHE14 history and its recent update.
We hope you will enjoy this issue of the Newsletter and we invite you to visit our newly update website at www.thew-project.org to learn about all new resources recently added to the website.
[1]Dessertenne F. La Tachycardie Ventriculaire a Deux Foyers Opposes Variables. Arch Mal Coeur 1966;59:263-72 [2] D'Alnoncourt CS et al. "Torsades de pointes" Arrhythmias. Re-entry or focal activity? Br Heart J 1982 48: 213-216. [3] Fabiato et al.Torsades de pointes, a quarter of a century later: A tribute to Dr. F. Dessertenne. Cardiovascular Drugs Therapy. vol 5, 1, 1991:167-169 [4] Moise, S. As Americans , we should get this right. Circulation. 1999, 100, 1462.
| |
ICH E14 Update by Borje Darpo, MD, PhD
The ICH E14 guidance on clinical evaluation of QT prolongation was adopted in May 2005 . An Implementation Working Group (IWG) was at the same time formed as recognition that this was an area of evolving science and accordingly there was a need for a continuous dialogue between regulators and industry. An ICH E14 website, to which questions could be submitted, was opened. Relatively few questions were submitted and it was not until June 2008 that the first Q&A document was released from the IWG ...
In June 2009 the ICH Steering Committee decided to disband the E14 IWG, close the website and to instead establish an Informal Discussion Group (IDG) with participants from both clinical (E14) and non-clinical disciplines (S7B). The decision was more political than science-based and emanated from sponsors who wanted to increase the relative importance of non-clinical studies and get away from the mandatory request of performing a TQT study for all compounds with systemic availability. The main question that the IDG was asked to look into was therefore whether accumulated experience with safety pharmacology studies and QT assessment in early studies would justify reopening the E14 document to substantially revise the requirement of a TQT study. Even before the first meeting of the group in St Louis in October 2010, it was clear that this idea did not have much support even among industry safety pharmacologist; most parties realize that FDA will only move on this topic based on convincing data and no such data have yet been compiled or presented. Based on some on-going, very interesting collaborative initiatives e.g. the ILSI/HESI Proarrhythmia initiative, data may eventually be compiled to support a move in this direction. This was also the conclusion of the St Louis meeting and ICH E14 will consequently not be reopened. The decision by the ICH Steering Committee to close the IWG group was also somewhat surprising, given that there are many outstanding issues with the E14 guidance on which both sponsors and regulators would benefit from clarification. Several such topics were discussed in St. Louis and the IDG agreed that there is an on-going need for further clarification. A proposal was therefore made that the IDG group would continue to meet and issue answers on relevant questions and this proposal was subsequently endorsed by the ICH Steering Committee. The following questions will therefore be addressed by the group... Read the full article here. |
| |
 |
The new version of the THEW website was released this first quarter 2010. As a new feature, we designed an easier access to all resources available on our servers. For instance, the website proposes a direct access to the updated descriptions of the
databases included in the warehouse, the ability to download all legal agreements for data sharing, for registering to the THEW, and for submitting research proposals. |
The beginning of year 2010 is marked by a series of presentations of the THEW initiative to several important organizations. First this week, a presentation co-authored by Dr. Lathrop (NHLBI), Dr. Stockbridge (FDA), Dr. Sanhai (FDA), and Dr. Boineau (NHLBI) will be presented at the Industry Event of the Clinical and Translational Science Awards at the NIH Campus in Washington DC. The objective of this presentation is to provide example of an "efficient and effective collaborative project" between government agencies, academic centers and industry. This presentation will be accompanied by a live demo of the THEW client application and its secured access to the warehouse databases. More information about this event.
Also in March, the Center and the THEW will be presented in Australia to the MedTeQ Centre of the School of Information Technology and Electrical Engineering at the University of Queensland in
St Lucia (Brisbane), as well as at the Department of Electrical Engineering at University of Melbourne.
Please visit the new webiste at www.thew-project.org
|
| |

Barbara J. Drew, RN, PhD, FAHA |
Hospital providers should be aware of rare, life-threatening heart rhythm by by B. Drew RN, PhD,FAHA
Cardiac arrest from a medication-induced heart rhythm problem is a rare but potentially catastrophic event in hospitalized patients, and hospital care providers need to be more aware of it according to a joint scientific statement from the American Heart Association and American College of Cardiology. The statement, published online in both Circulation: Journal of the American Heart Association and Journal of the American College of Cardiology, is also endorsed by the International Society for Computerized Electrocardiology and the American Association of Critical-Care Nurses.
The rhythm disturbance, called Torsades de Pointes (TdP), has a characteristic electrocardiogram pattern described as a “twisting” of points on the graphic recording. This abnormal rhythm can degenerate into an even more serious rhythm disturbance called ventricular fibrillation, which then causes sudden cardiac arrest.
“This scientific statement is particularly important for healthcare professionals who administer QT-prolonging drugs in hospital units where patients have continuous ECG monitoring such as intensive care and telemetry units.... Read the full article here.
|
| |
THEW Team: Betty Mykins, Technical Associate
Betty Jane Mykins is a native of Rochester N.Y. and attended Monroe Community College. She has twenty years of Cardiology experience at area hospitals and is an electrocardiograph technician in rhythm interpretation. Betty Jane is a study coordinator for the ICD LQTS registry and for several EPA projects. Her primary roles in the THEW organization are to prepare incoming Holter ECGs and make annotations, upload data files, run software scanning programs, and monitor compliance with HIPPA requirements. |

Betty Mykins, THEW Technical Associate |
| |
|